Ch. 1.3 Scenarios to Review for Scientific Method CLICK HERE
Chapter 2.1


Section 2.1
Atom: the smallest basic unit of matter
How to read the famous

| Element | Percentage | Mass [Kg] | Cost/Kg | Value | |
|---|---|---|---|---|---|
| O | Oxygen | 65 | 49.14 | $0.20 | $9.83 |
| C | Carbon | 18 | 18.29 | $0.01 | $0.18 |
| H | Hydrogen | 10.2 | 8.00 | $0.20 | $1.60 |
| N | Nitrogen | 3.1 | 2.06 | $0.20 | $0.41 |
| Ca | Calcium | 1.6 | 1.14 | $0.10 | $0.11 |
| P | Phosphorus | 1.2 | 0.89 | $1.00 | $0.89 |
| K | Potassium | 0.25 | 0.16 | $650.00 | $104.00 |
| S | Sulfur | 0.25 | 0.16 | $0.10 | $0.02 |
| Na | Sodium | 0.15 | 0.11 | $250.00 | $28.57 |
| Cl | Chlorine | 0.15 | 0.11 | $1.00 | $0.11 |
| Mg | Magnesium | 0.05 | 0.02 | $3.00 | $0.07 |
| Fe | Iron | 6.00E-03 | 4.80E-03 | $0.20 | $0.00 |
| F | Fluorine | 3.70E-03 | 2.97E-03 | $2,000.00 | $5.94 |
| Zn | Zinc | 3.20E-03 | 2.63E-03 | $2.00 | $0.01 |
| Si | Silicon | 2.00E-03 | 1.14E-03 | $2.00 | $0.00 |
| Rb | Rubidium | 4.60E-04 | 7.77E-04 | $10,000.00 | $7.77 |
| Sr | Strontium | 4.60E-04 | 3.66E-04 | $1,000.00 | $0.37 |
| Br | Bromine | 2.90E-04 | 2.97E-04 | $50.00 | $0.01 |
| Pb | Lead | 1.70E-04 | 1.37E-04 | $0.40 | $0.00 |
| Cu | Copper | 1.00E-04 | 8.23E-05 | $7.00 | $0.00 |
| Al | Aluminium | 8.70E-05 | 6.86E-05 | $2.00 | $0.00 |
| Cd | Cadmium | 7.20E-05 | 5.71E-05 | $10.00 | $0.00 |
| Ce | Cerium | 5.00E-05 | 4.57E-05 | $50.00 | $0.00 |
| Ba | Barium | 3.10E-05 | 2.51E-05 | $60.00 | $0.00 |
| Sn | Tin | 2.40E-05 | 2.29E-05 | $20.00 | $0.00 |
| I | Iodine | 1.60E-05 | 2.29E-05 | $2.00 | $0.00 |
| Ti | Titanium | 1.30E-05 | 2.29E-05 | $8.00 | $0.00 |
| B | Boron | 6.90E-05 | 2.06E-05 | $6,000.00 | $0.12 |
| Se | Selenium | 1.90E-05 | 1.71E-05 | $53.00 | $0.00 |
| Ni | Nickel | 1.40E-05 | 1.71E-05 | $20.00 | $0.00 |
| Cr | Chromium | 2.40E-06 | 1.60E-05 | $20.00 | $0.00 |
| Mn | Manganese | 1.70E-05 | 1.37E-05 | $5.00 | $0.00 |
| As | Arsenic | 2.60E-05 | 8.00E-06 | $5.00 | $0.00 |
| Li | Lithium | 3.10E-06 | 8.00E-06 | $100.00 | $0.00 |
| Hg | Mercury | 1.90E-05 | 6.86E-06 | $40.00 | $0.00 |
| Cs | Caesium | 2.10E-06 | 6.86E-06 | $3,000.00 | $0.02 |
| Mo | Molybdenum | 1.30E-05 | 5.71E-06 | $300.00 | $0.00 |
| Ge | Germanium | 1.30E-05 | 5.71E-06 | $2,000.00 | $0.01 |
| Co | Cobalt | 2.10E-06 | 3.43E-06 | $200.00 | $0.00 |
| Sb | Antimony | 1.10E-05 | 2.29E-06 | $300.00 | $0.00 |
| Ag | Silver | 1.00E-06 | 2.29E-06 | $700.00 | $0.00 |
| Nb | Niobium | 1.60E-04 | 1.71E-06 | $180.00 | $0.00 |
| Zr | Zirconium | 6.00E-04 | 1.14E-06 | $600.00 | $0.00 |
| La | Lanthanum | 2.00E-04 | 9.14E-07 | $1,500.00 | $0.00 |
| Te | Tellurium | 1.20E-05 | 8.00E-07 | $240.00 | $0.00 |
| Ga | Gallium | 1.20E-05 | 8.00E-07 | $1,800.00 | $0.00 |
| Y | Yttrium | 1.20E-05 | 6.86E-07 | $2,000.00 | $0.00 |
| Bi | Bismuth | 1.20E-05 | 5.71E-07 | $400.00 | $0.00 |
| Tl | Thallium | 1.40E-05 | 5.71E-07 | $500.00 | $0.00 |
| In | Indium | 1.40E-05 | 4.57E-07 | $2,000.00 | $0.00 |
| Au | Gold | 1.40E-05 | 2.29E-07 | $40,000.00 | $0.01 |
| Sc | Scandium | 1.40E-05 | 2.29E-07 | $10,000.00 | $0.00 |
| Ta | Tantalum | 1.40E-05 | 2.29E-07 | $200.00 | $0.00 |
| V | Vanadium | 2.60E-05 | 1.26E-07 | $50.00 | $0.00 |
| Th | Thorium | 2.00E-07 | 1.14E-07 | $0.00 | |
| U | Uranium | 1.30E-07 | 1.14E-07 | $0.00 | |
| Sm | Samarium | 5.00E-09 | 5.71E-08 | $0.00 | |
| W | Tungsten | 5.00E-09 | 2.29E-08 | $0.00 | |
| Be | Beryllium | 5.00E-09 | 4.11E-08 | $0.00 | |
| Ra | Radium | 1.00E-17 | 0.00E+00 | $0.00 |
Summing up all the amounts in the Value column, we come to a grand total of just over $160.
You'd better keep eating those bananas!*
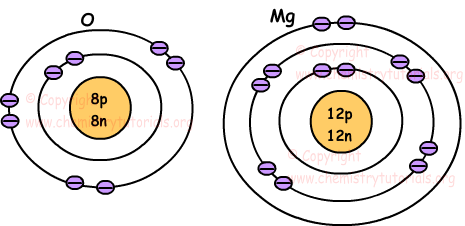
All atoms have a similar structure. Each are composed of protons, neutrons, and electrons.
All atoms contain negatively charged particles known as ELECTRONS. These particles are found in regions around a nucleus known as Energy Levels.
The outermost energy level (valence electrons) determine how atoms form ions or form covalent bonds
Atoms can come together through different connections to make compounds (atoms of different elements bonded together in certain ratios ).....


using these types of bonds..........(both are strong bonds)

Ions are charged particles (+ or -)
1st Type of Bond
Ionic Bond: forms between oppositely charged ions
Positive ions—formed when atoms lose electrons
Negative ions—formed when atoms gain electrons
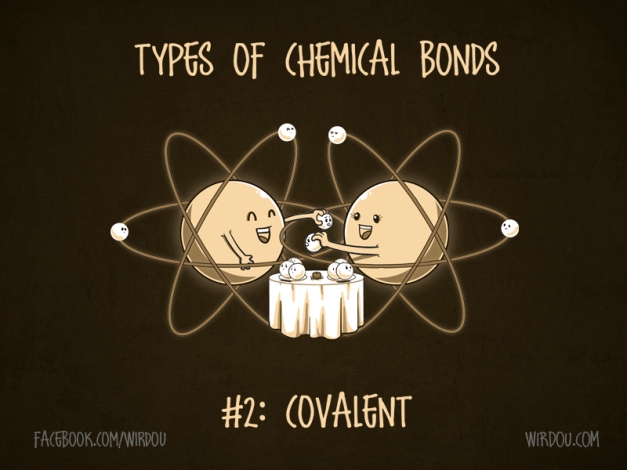
2nd Type of Bond
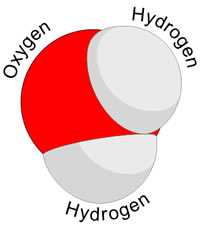
Covalent Bond: form when 2 atoms share their electrons
Molecules two or more atoms held together by covalent bonds
These are some of the most important atoms....








No comments:
Post a Comment