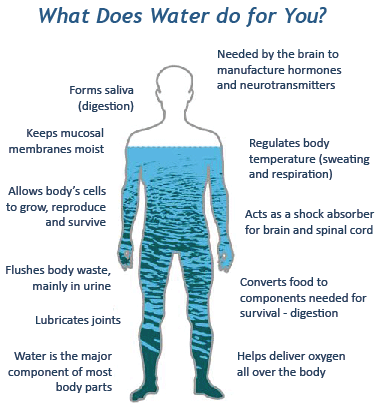
H2O Vital 4 LIFE!!
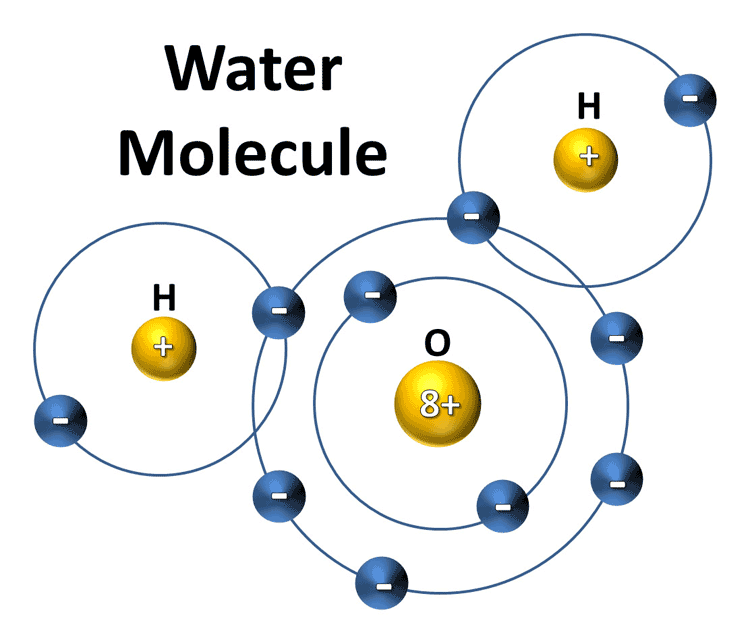
Structure of Water....

How do fish survive a cold winter if their pond freezes? Most substances contract when frozen, water expands. Water is less dense as a solid (ice) than as a liquid. It acts as an insulator that allows water underneath to remain a liquid.
What is the charge of water?
Water is known as a polar molecule because it resembles a magnet with opposite poles (charges) being on opposite sides. This allows water to form bonds with other water molecules. Remember opposites attract.
Properties of water....
Hydrogen bonds give water a high specific heat. This is an important property to have. Inside cells this property allows high energy chemical reactions to happen because these reactions produce a great amount of heat. Water absorbs this heat and helps regulate internal cell temperatures. Hydrogen bonds are formed from opposite charges of a polar molecule, like in the case of water.
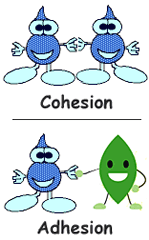
Cohesion: Water molecules sticking to water molecules.
Adhesion: Water molecules sticking to different source.
Many compounds dissolve in H2O....
Important material such as sugar and oxygen cannot be transported from one part of the organism to another without the help of a water based fluid.
Blood is made up of 95% water and carries important material all over your body.
Vocabulary to know
Solute: substance being dissolved

Solvent: substance that dissolves a solute. There is more of a solvent than a solute in a solution.

Solution: homogeneous substance formed from the dissolved solute and solvent mixture.

Where can we use this knowledge....
Some compounds release a H+ when dissolved in water (acid)
Other compounds remove a H+ from a solution (base)

Acids pH:1-6; release a H+
Neutral pH:7; neither acidic nor basic
Basic pH:8-14; remove a

No comments:
Post a Comment